Current Science
Current Science
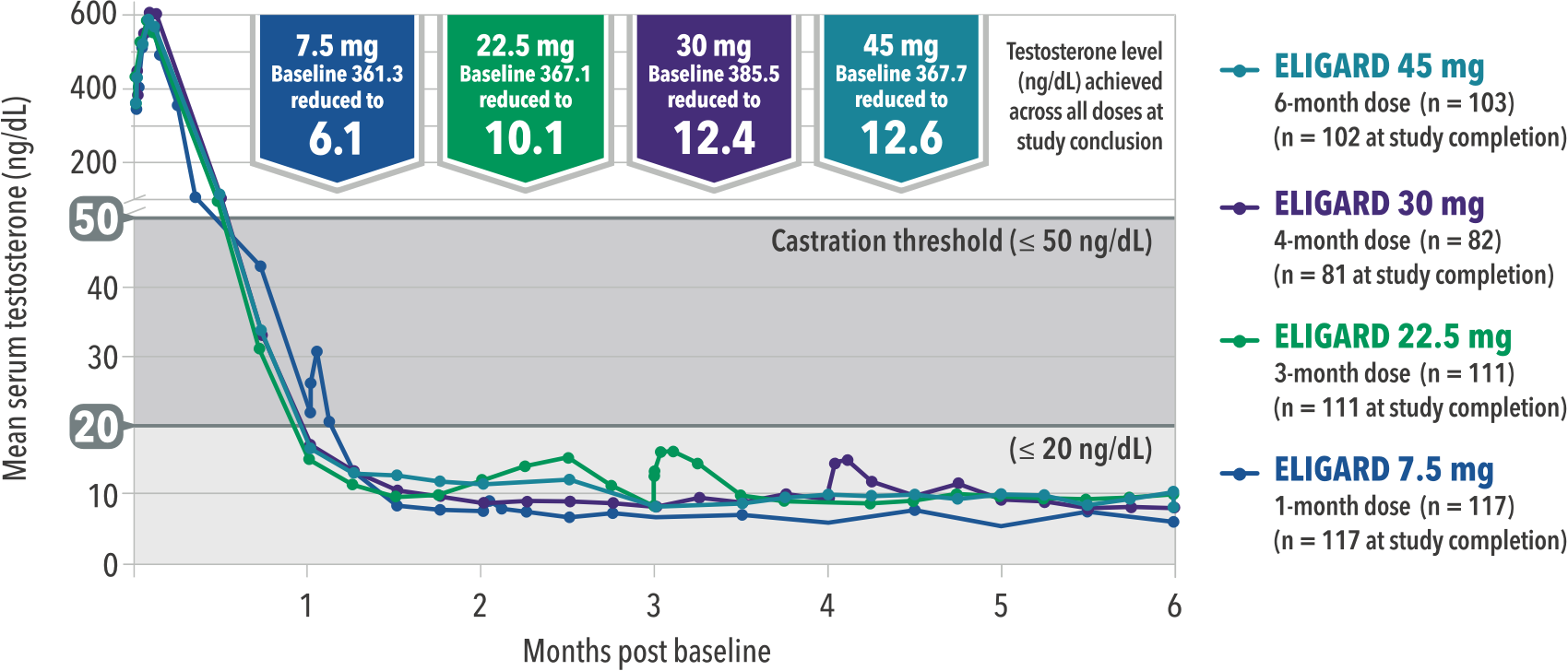
ELIGARD® achieves and maintains low testosterone levels across all four doses, including the longest duration available (six-month dose). [1]

Testosterone target levels
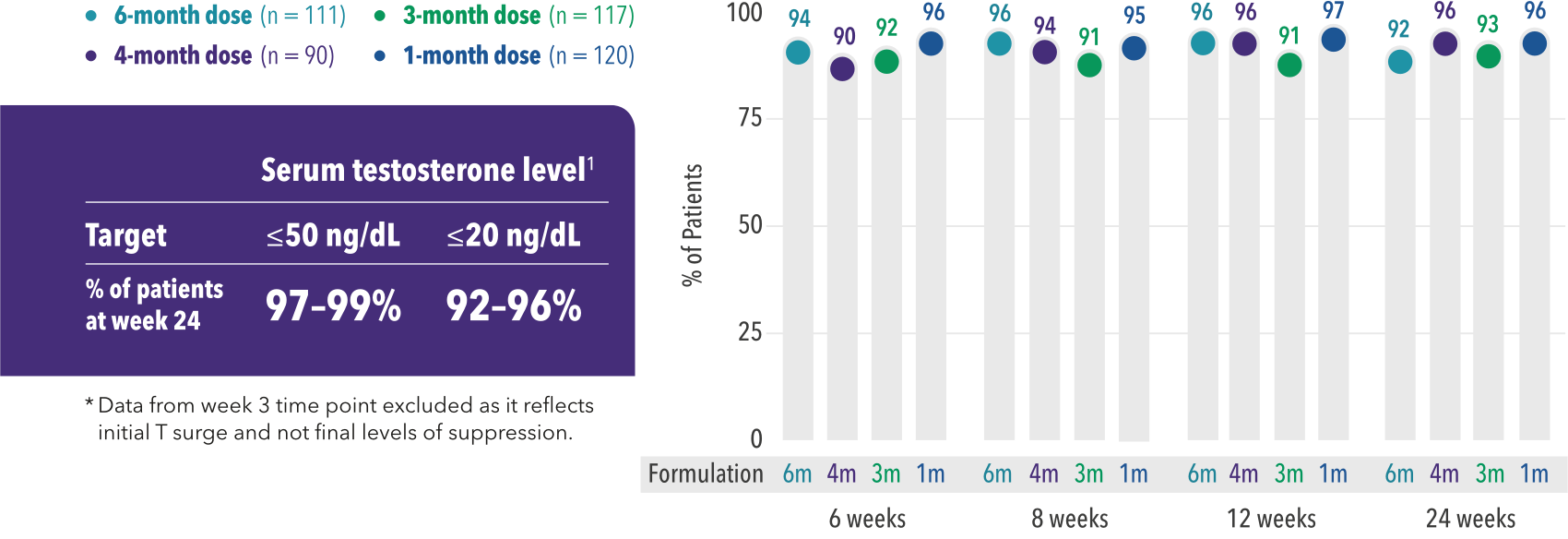

Current science reflects testosterone target levels for castration of ≤20 ng/dL. [2]
Achieving and maintaining low testosterone (T) to surgical castration level is the goal of androgen deprivation therapy (ADT) for advanced prostate cancer.
ELIGARD achieves low testosterone levels across all doses that reflects current science. [1]
Current assay methodology shows testosterone (T) level post-surgical castration is ≤20 ng/dL. [2]
Nadir T levels
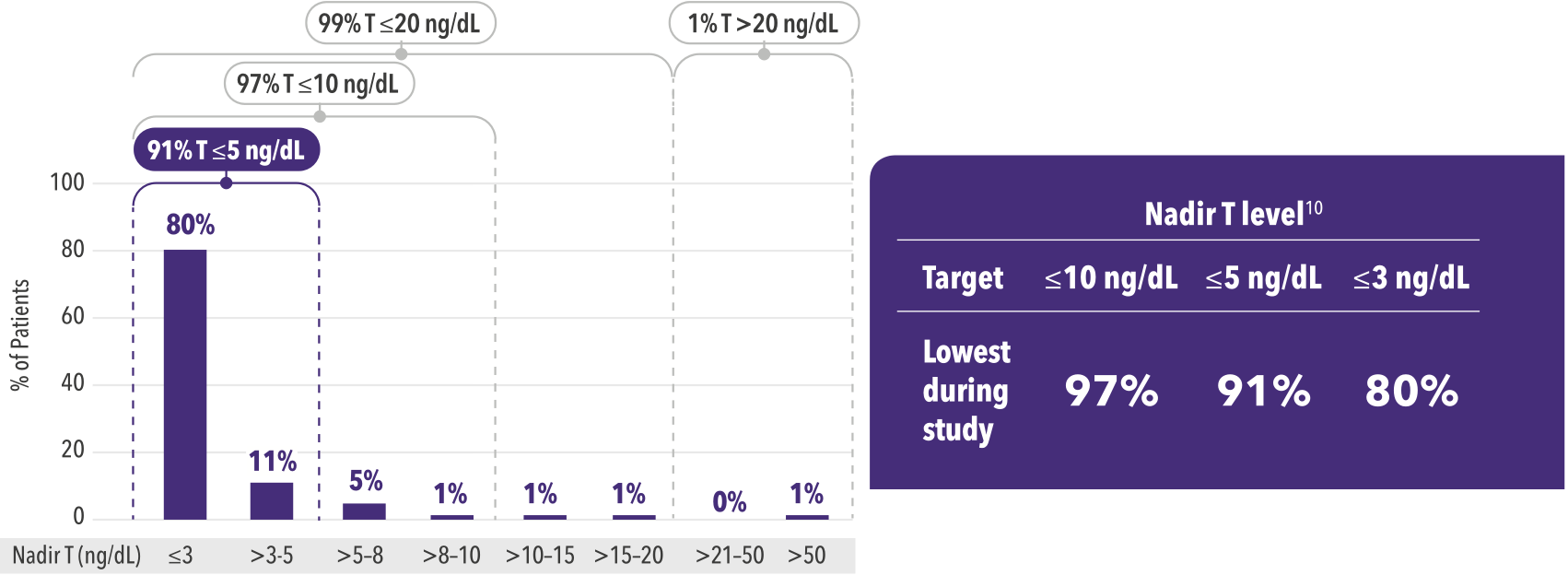
Current science considers nadir T levels. Nadir serum testosterone or nadir T is the lowest value during androgen deprivation therapy (ADT), and evidence is accumulating for nadir T levels around prostate cancer treatments. [3] [4] [5] [6] [7] [8]
ELIGARD® achieved low nadir T levels across all pivotal trials. Nadir T of ≤5 ng/dL was achieved in 91% of patients. [3]
Serum PSA
| Dose | 7.5 mg | 22.5 mg | 30 mg | 45 mg |
| Mean PSA reduction at study conclusion | 94% | 98% | 86% | 97% |
| Patients with normal PSA at study conclusion | 94% | 91% | 93% | 95% |
91% to 95% of patients achieved normal serum PSA values at study conclusion. [1]
In all studies, ELIGARD decreased serum PSA in all patients who had elevated levels at baseline.
References
- ELIGARD® (leuprolide acetate) for injectable suspension, 7.5 mg, 22.5 mg, 30 mg, 45 mg prescribing information. Fort Collins, CO: Tolmar Therapeutics, Inc.; 2019
- Shore N, et al., BJU Int 2016.
- Pieczonka C, et al., Rev Urol 2018
- Bryant AK, McKay RR, Kader AK, Parsons JK, Einck JP, Kane CJ, Mundt AJ, Murphy JD, Rose BS, Sub-castrate testosterone nadir and clinical outcomes in intermediate or high-risk localized prostate cancer, International Journal of Radiation Oncology • Biology • Physics (2019), doi: https://doi.org/10.1016/j. ijrobp.2018.12.001
- Suzman, Antonarakis, J Clin Oncol 2016
- Klotz, et al., J Clin Oncol 2015
- Kamada, et al., J Urol 2015
- Nabid et al., Eur Urol Suppl 2017