Description
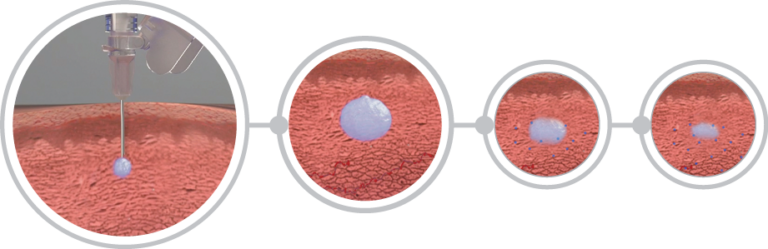
Effective testosterone suppression
 zoom
zoom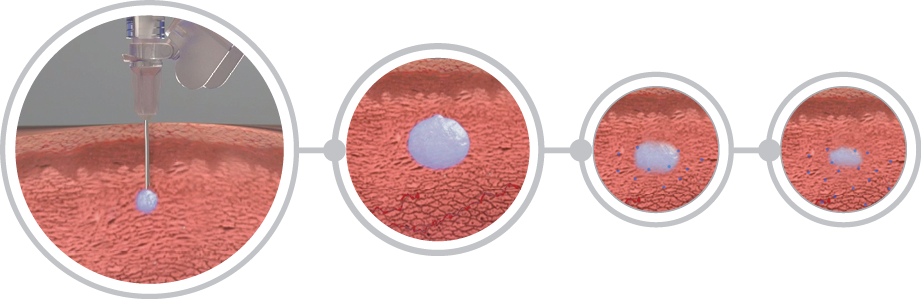
Effective testosterone suppression
ELIGARD’s innovative in-situ polymeric gel extended delivery system provides controlled drug release enabling consistent suppression of testicular testosterone synthesis throughout the dosing period. [1]
Learn more about ELIGARD’s Clinical Data here.
Clinical DataFlexible dosing
 zoom
zoom
Flexible dosing
ELIGARD offers four (4) dosing options to meet different patient needs. [2]
Learn more about ELIGARD’s Dosage Options here.
Dosage OptionsEnhanced patient experience
 zoom
zoom
Enhanced patient experience
ELIGARD’s subcutaneous injection allows for numerous injection sites contributing to the patient’s comfort level. [3]
Benefits of subcutaneous (SC) injection include:
For patients…
- Is safe for people taking anticoagulants as a short needle should not penetrate the muscle
- Provides a wide range of injection site options
- Avoids muscle soreness at injection site
- Is suitable for patients with little muscle mass
- Has a low risk of bone or nerve damage due to needle trauma
For health care professionals…
- Allows for flexibility in site of care — can be administered in semi-private settings (e.g., infusion center)
- Is easy to administer to patients in wheelchairs
- Is safe for patients who are on anticoagulants as a short needle should not penetrate the muscle
- Is suitable for patients with little muscle mass
- Has a low risk of bone or nerve damage due to needle trauma
Structural formula
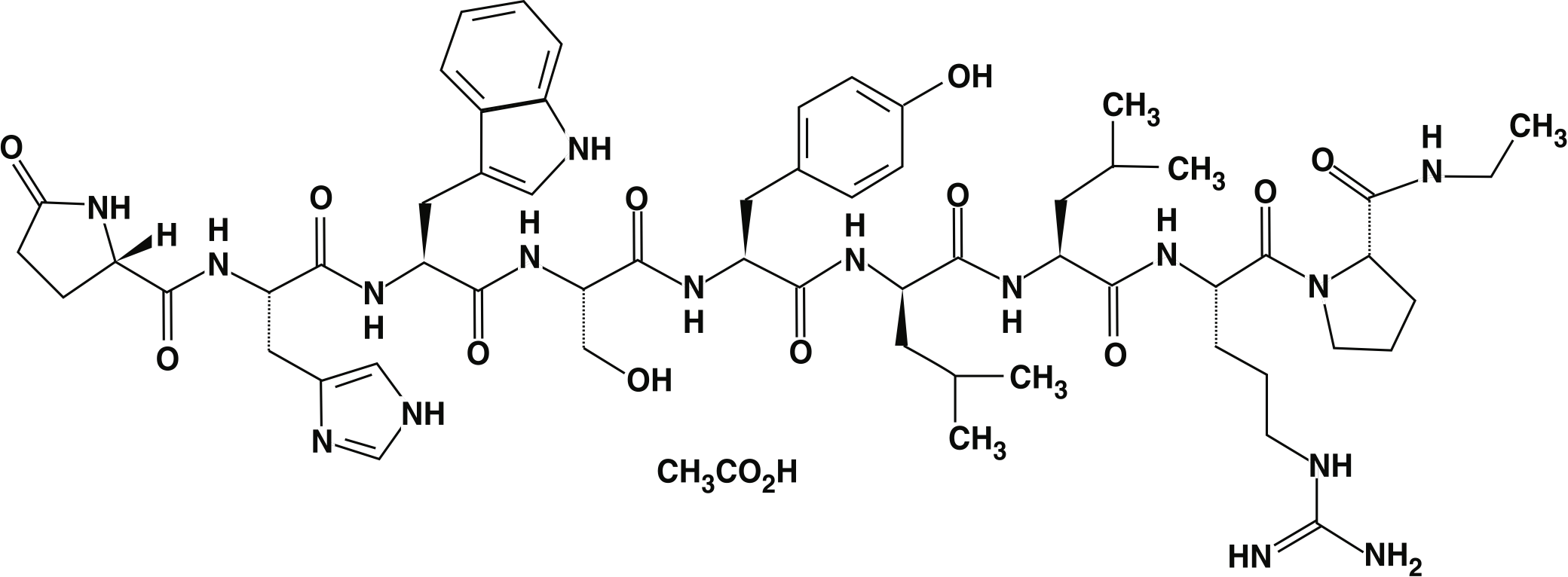

Leuprolide acetate has been used to treat patients with advanced prostate cancer for over 30 years. Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH).
When given continuously, it:
- inhibits pituitary gonadotropin secretion
- suppresses testicular steroidogenesis.
The analog possesses greater potency than the natural hormone.
The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt).
USP <800> considerations
USP General Chapter <800> Hazardous Drugs—Handling in Healthcare Settings is only applicable in the context of compounding. ELIGARD is a conventionally manufactured product, and preparation in accordance with the approved labeling for administration to an individual patient is not considered compounding. Therefore, USP <800> does not apply to the mixing and administration of ELIGARD.
FDA approval
ELIGARD is indicated for the palliative treatment of advanced prostate cancer. The one-month, three-month, four-month, and six-month formulations received FDA approval in Jan 2002, July 2002, Feb 2003 and Dec 2004, respectively.
References
- Sartor O. Eur Urol 2006
- ELIGARD® (leuprolide acetate) for injectable suspension 7.5 mg, 22.5 mg, 30 mg, 45 mg prescribing information. Fort Collins, CO: Tolmar Therapeutics, Inc.; 2019.
- Prettyman J. Urologic Nursing 2019